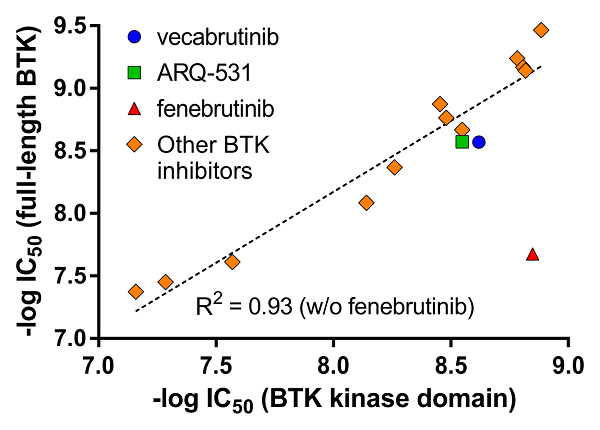
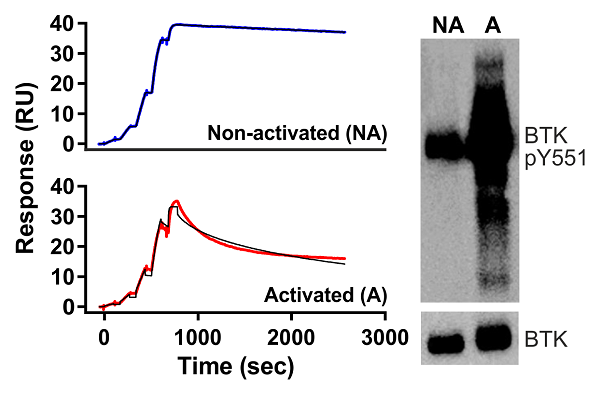
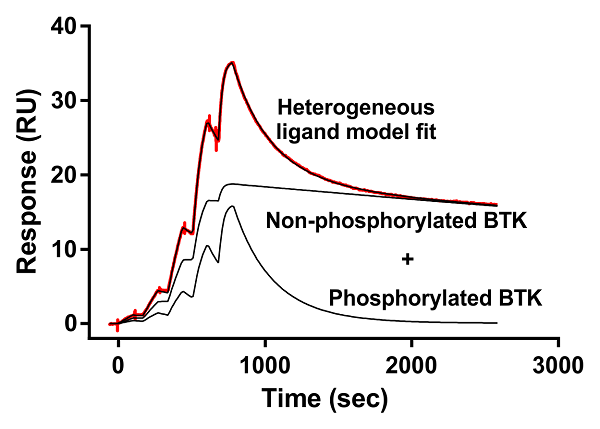
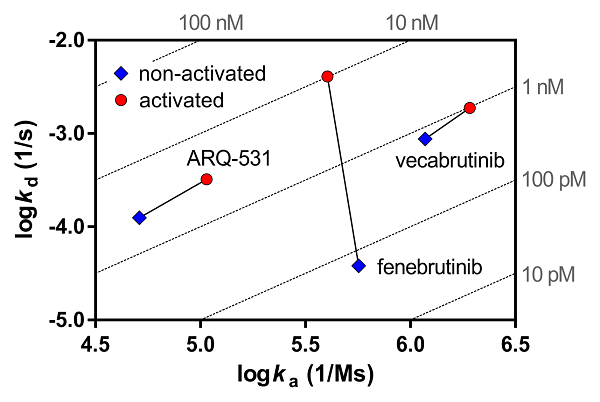
Phosphorylation state-dependent binding kinetics of BTK inhibitor fenebrutinib
Elucidating the selectivity of inhibitors for specific kinase phosphorylation states
References
Dinh et al. (2007) Activation mechanism and steady state kinetics of bruton’s tyrosine kinase. The Journal of Biological Chemistry 282 (12):8768-8776.
Crawford et al. (2018) Discovery of GDC-0853: a potent, selective, and noncovalent bruton’s tyrosine kinase inhibitor in early clinical development. Journal of Medicinal Chemistry, 61 (6):2227-2245.