IL4I1 in ascites as a marker in high-grade serous ovarian cancer
The emergence of immunotherapy has revolutionized cancer treatment for various malignancies. However, immunotherapies have demonstrated only limited efficacy in ovarian cancer, which may be explained by the highly immunosuppressive nature of the ovarian tumor microenvironment. Enzymes that catabolize amino acids critical for adequate T cell responses, such as tryptophan, or produce metabolites that inhibit immune cell function may contribute to this immunosuppressive environment.
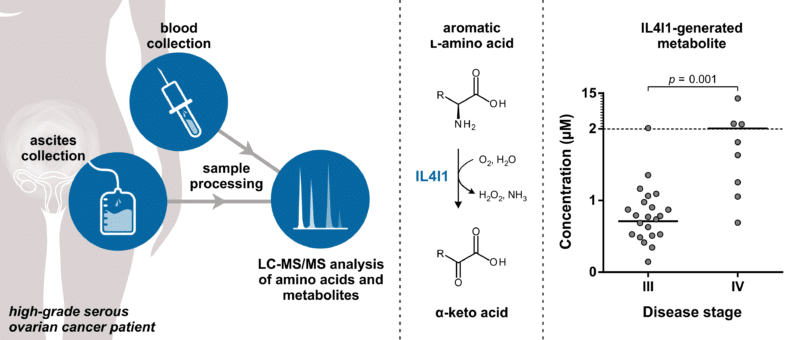
In a recent paper published in Cancers,1 Yvonne Grobben from Oncolines investigated the role of various immunosuppressive amino acid-metabolizing enzymes in high-grade serous ovarian cancer. Through targeted liquid chromatography–tandem mass spectrometry (LC-MS/MS)-based metabolomics, the activity of well-known immunotherapeutic drug targets, such as indoleamine 2,3-dioxygenase (IDO1) and arginase 1 (ARG1), was measured in plasma and ascites samples collected from patients with advanced stages of the disease. Ascites is the abnormal buildup of fluid frequently found in the abdomen of women with ovarian cancer. The levels of metabolites produced by the l-amino acid oxidase ‘interleukin 4 induced 1’ (IL4I1) were also measured. IL4I1 was recently proposed to play a role in resistance to IDO1 inhibitor therapy.2
By comparing amino acid and metabolite levels in plasma and ascites, Grobben showed that ascites is a valuable source of biomarkers related to amino acid-derived metabolites. High levels of IL4I1-associated metabolites were found in this fluid and correlated significantly with the concentration of secreted IL4I1. Moreover, levels of these metabolites were elevated in patients with stage IV compared to stage III disease, suggesting an involvement of IL4I1 in disease progression. Analysis of pleural effusions from non-small cell lung cancer patients additionally indicated that enhanced activity of IL4I1 is not limited to ovarian cancer, suggesting a potential role for this enzyme across cancer types. The studies provide support for IL4I1 as a potential novel small molecule drug target for cancer immunotherapy. Moreover, the described LC-MS/MS methods can be used for evaluation of biomarkers for patient stratification and measurement of target engagement of IL4I1 inhibitors, which are currently in development.3
The study was performed in collaboration with Utrecht University and Radboud University Medical Center (Radboudumc, Nijmegen) in the Netherlands, and Pangaea Oncology, S.A. in Barcelona (Spain). Samples from ovarian cancer patients were collected by researchers from Radboudumc in a project supported by Foundation Ruby and Rose. Lung cancer pleural effusions were provided by Pangaea Oncology, with whom Oncolines collaborates in a Eurostars-funded project.
References
- Grobben Y, den Ouden JE, Aguado C, van Altena AM, Kraneveld AD, Zaman GJR (2023) Amino Acid-Metabolizing Enzymes in Advanced High-Grade Serous Ovarian Cancer Patients: Value of Ascites as Biomarker Source and Role for IL4I1 and IDO1. Cancers, 15(3):893.
- Sadik et al. (2020) IL4I1 Is a Metabolic Immune Checkpoint That Activates the AHR and Promotes Tumor Progression. Cell, 182(5):1252–1270.
- MacKinnon et al. (2020) Anti-tumor activity of CB-668, a potent, selective and orally bioavailable small-molecule inhibitor of the immuno-suppressive enzyme Interleukin 4 (IL-4)-Induced Gene 1 (IL4I1). J Immunother Cancer, 8(Suppl 3):A747.
Oncolines B.V. is a precision medicine services company in oncology and cancer immunotherapy. Oncolines is part of the Symeres group of companies, a group of high-quality CROs and CDMOs based in Europe and the United States.