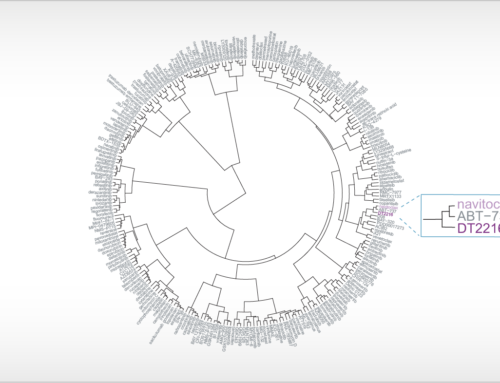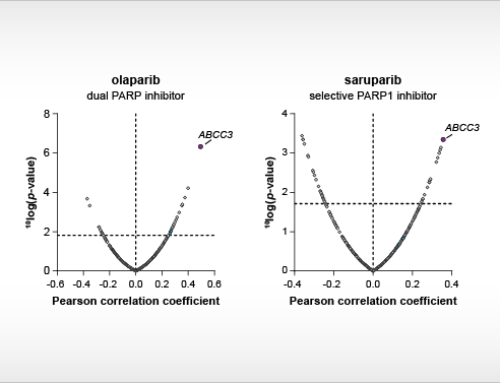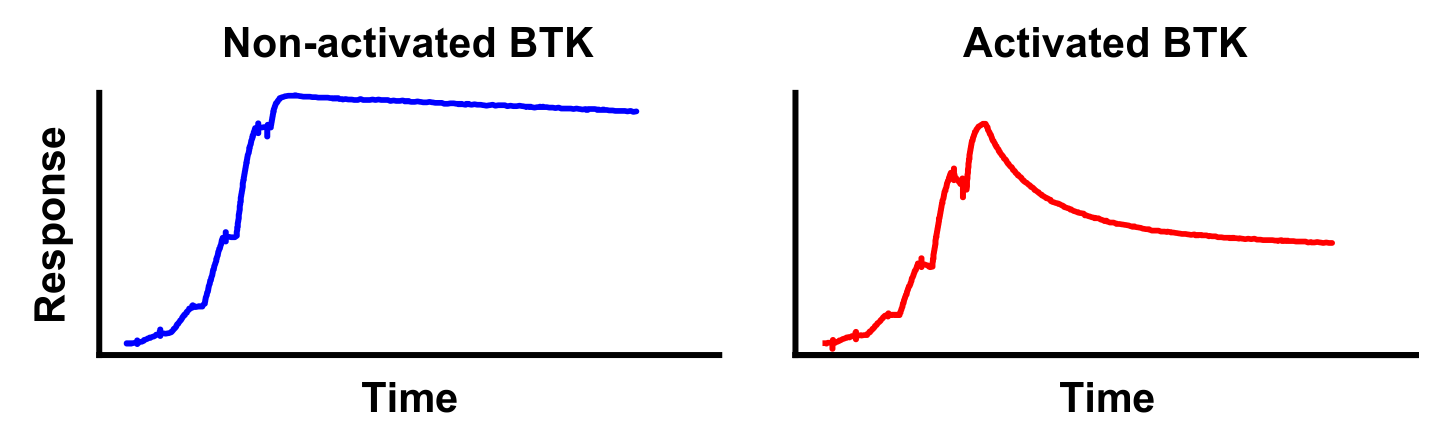
April 28, 2021: A new case study is presented going in-depth on the biochemical properties of Bruton’s tyrosine kinase (BTK) inhibitors. The figure shows surface plasmon resonance sensorgrams for the binding of the BTK inhibitor fenebrutinib on non-activated BTK (left) and activated BTK (right).
Phosphorylation status determines kinase inhibitor binding
A new case study is presented on our ResidenceTimer™ website going in-depth on the biochemical properties of Bruton’s tyrosine kinase (BTK) inhibitors using enzymatic activity assays and surface plasmon resonance (SPR). Using these techniques, the clinical stage inhibitor fenebrutinib is shown to have a unique preference for binding to non-phosphorylated BTK compared to its phosphorylated counterpart. Key features of this case study include the comparison of full-length BTK and the BTK kinase domain in enzymatic assays, which differ in their ability to be autophosphorylated, and the comprehensive analysis of SPR binding curves using an alternative binding model.