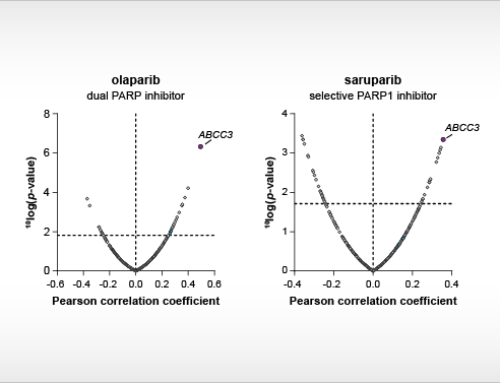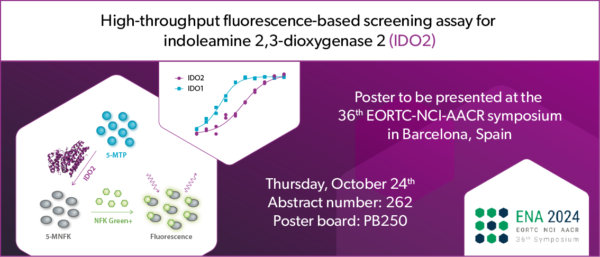
The tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase 2 (IDO2) is a potential therapeutic target for long COVID and cancer immunotherapy. IDO2 has 44% amino acid identity with IDO1, which has intensively been investigated to increase the efficacy of immune checkpoint inhibitor therapy for cancer. However, a phase III clinical trial of the IDO1 inhibitor epacadostat with the anti-PD1 antibody pembrolizumab (Keytruda®) was terminated prematurely due to an absence of clinical benefit of the combination over pembrolizumab plus placebo [1]. Different explanations for this clinical failure have been raised, including a potential role of IDO2 [2]. Recently, IDO2 was found to be expressed in lung, heart, brain and blood cells from patients with severe COVID infections, indicating IDO2 as a potential therapeutic target for long COVID [3]. Oncolines researchers have modified a fluorescence-based assay that they previously developed and used for high-throughput screening of IDO1 and TDO [4-6], to enable biochemical screening for small molecule inhibitors of IDO2. In collaboration with colleagues from Symeres, an improved fluorescent probe was developed for this assay, termed NFK Green⁺.
Oncolines commercializes the IDO2 NFK Green⁺ assay as a kit for 1,000 or 10,000 data points. Each kit contains IDO2 protein, assay buffer and NFK Green⁺ assay reagent, and is available to clients worldwide. The new product will be launched at the EORTC-NCI-AACR (ENA) conference, which will be held in Barcelona (Spain) from October 23rd to 25th. On Thursday, Dr. Guido Zaman, Managing Director of Oncolines, will present a poster in the session Immune Checkpoints, entitled ‘High-throughput fluorescence-based screening assay for indoleamine 2,3-dioxygenase 2 (IDO2)’ at poster board PB250.
References
- Long et al. (2019) Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncology 20, 1083–97.
- Van den Eynde et al. (2020) Is there a clinical future for IDO1 inhibitors after the failure of epacadostat in melanoma? Annual Review of Cancer Biology 4, 241–56.
- Guo et al. (2023) Prolonged indoleamine 2,3-dioxygenase-2 activity and associated cellular stress in post-acute sequelae of SARS-CoV-2 infection. eBioMedicine 94, 104729.
- Seegers et al. (2014) High-throughput fluorescence-based screening assays for tryptophan-catabolizing enzymes. Journal of Biomolecular Screening 19, 1266–74.
- Perez-Pardo et al. (2021) Pharmacological validation of TDO as a target for Parkinson’s disease. The FEBS Journal 288, 4311–31.
- Grobben et al. (2021) Targeting indoleamine 2,3-dioxygenase in cancer models using the novel small molecule inhibitor NTRC 3883-0. Frontiers in Immunology 11, 609490.
Oncolines B.V. is a precision medicine services company in oncology and cancer immunotherapy. Oncolines is part of the Symeres group of companies, a group of high-quality CROs and CDMOs based in Europe and the United States.